Lime is calcium bearing inorganic mineral obtained from burnt (calcined) limestone, composed mainly of calcium oxide (CaO) and/or calcium hydroxide (Ca(OH)2).
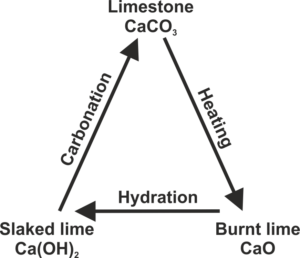
The rocks and minerals from which these materials are derived, typically limestone or chalk, are composed primarily of calcium carbonate.
Burning (calcination) of these minerals in a lime kiln converts them into the highly caustic material burnt lime (calcium oxide, CaO).
CaCO3 + Heat ⟶ CaO + CO2
Burnt lime is a commodity used in large scales, for example in steel fabrication, as base material for construction materials, etc.
For construction materials like mortar, burnt lime is combined with water (hydrated) into less caustic (but still strongly alkaline) slaked or hydrated lime (calcium oxide Ca(OH)2)
CaO + H2O ⟶ Ca(OH)2
The process in which limestone (calcium carbonate) is converted to burnt lime by heating, then to slaked lime by hydration, and finally naturally reverts to calcium carbonate by carbonation is called the lime cycle.
A typical example is the use of the mortar for building purposes. Carbon dioxide (e.g. from the atmosphere or aggregates) is reacting with hydrated lime to convert it back to limestone.
Ca(OH)2 + CO2 ⟶ CaCO3 + H2O.
Lime is among the oldest material used by humans. Lime and limestone products are more in demand today than ever before in history as they also serve as an essential component in various industrial processes.
Today, lime is used:
- In domestic products like dry bleaches, glass, gold, garden neutralizers, leather, masonry, mortar, paint, paper, sugar, toothpaste etc.
- For the environment preservation, air pollutant reduction, epidemic diseases control, waste-water treatment, sewage treatment.
- In production industries for alumina, barium, glass, lithium, magnesium, nickel, petroleum refining, silver, steel, uranium, etc.